DIAGNOSTICS
The analysis of mitochondrial disease involves the integration of different medical specialties organized in subsequent stages. The results of laboratory and instrument analysis are integrated with clinical examination, family and natural history of the patient.
The clinical evaluation of children with suspected mitochondrial pathology is carried out in the Metabolic Unit of the Division of Child Neurology at the National Neurological Institute.
The clinical evaluation of adult patients is carried out by Dr. Costanza Lamperti and colleagues of the Molecular Neurogenetics Unit (for more details see OUR SERVICES).
These diseases strike primarily the brain and skeletal muscle. Therefore neuroradiological evaluation based on brain Magnetic Resonance Imaging (MRI) and/or mass spectrometry, and neurophysiological studies including Evoked Potentials and EEG are routinely performed. However, the most fundamental exam in most cases is the muscle biopsy, which is used for morphological studies by light and electronic microscopy and provides the biological material indispensable for biochemical analysis of the respiratory chain and for mutation analysis on candidate genes. Muscle biopsy is particularly useful in pediatric cases, in which symptoms are often unspecific and it is therefore not possible to get a diagnosis on the basis of of clinical examination only.
All of the exams here reported, helpful for the diagnosis of mitochondrial disease patients, are carried out at our Institute.
Metabolic screening for suspected mitochondrial disease| Assay | Sample* | Comments |
| Glucose | B | |
| Ions and electrolytes | B | |
| Lactata, Pyruvate, Alanine | B, CSF | Do not use tourniquet; intercurrent infections, exercise and stress can alter the results |
| Lactate/Pyruvate ratio | B | Useful to determine the point of the metabolic block |
| Ammonia | B | |
| Aminoacids | B,U, CSF | Urinary aminoacids can be collected during fast conditions or after a meal. Generalized aciduria can suggest a mitochondriopathy |
| Organic acids | U | Take a 24h urine collection. Keep urine a t 4°C in sterile conditions with no preservants |
| Carnitine and derivatives | B | Free, short-chain esters, long-chain esters, and total carnitine should be measured separately |
| Ketones | B,U | Should be measured during a metabolic crisis |
Muscle Biopsy
It is now carried out with the use of a surgical needle, which is less painful
and less invasive then the "open" surgical technique. Needle biopsy
is carried out under local anesthesia in adult patients and under general
anesthesia in children. Suture stitches are not necessary, the resulting scar
is very small and leaves no lasting effect on the biopsied muscle. It is of
utmost importance that the exam is carried out in a specialized center, which
can guarantee the correctness and safety of the technique, the proper
conservation of the material and the completeness of the analysis.
The following investigations can be carried out on muscular tissue after the biopsy has been done:
In particular cases, electron microscope examination can visualize the
mitochondrial internal structure, which may be abnormal in mitochondrial
disorders.
Skin Biopsy
Cell culture of fibroblasts obtained from skin biopsy can be useful in many
cases:
Our center has a unique patrimony in Italy of more than 2000 lines of fibroblasts from patients with congenital metabolic pathologies. These cell lines, together with >7000 DNA samples, are stored in the biobank “Cell line and DNA Bank of Genetic Movement Disorders and Mitochondrial Diseases” part of the Network of Telethon Genetic Biobank and Eurobiobank.
Genetic analyses
The step following the clinical evaluation and indications from instrumental and biochemical exams is the genetic analysis. Biochemical assays and molecular investigations are usually carried out consecutively, thus resulting tobe time consuming and expensive. Several months are required for the diagnostic process to be completed. In addition, near 50% of patients remains without a final genetic diagnosis. The incomplete knowledge we have about the physio-pathological processes underpinning these disorders makes every protein in the cell to be - potentially - the cause of the disease. The identification of the genetic cause is considered, for inherited disorders, the final classification of the disease (which put an end to the so-called “diagnostic odyssey” of patients with rare disease).
The diagnostic genetic portfolio offered by our Unit comprises molecular exams for mitochondrial DNA (including the sequencing of the entire mtDNA and qualitative/quantitative analyses) and known nuclear genes associated with mitochondrial disorders, analyzed by direct sequencing.
The priority and the type of analysis to be carried out
are established by clinicians and geneticists, after they have evaluated –
together – all the available documentation (clinical presentation,
neuroradiological pattern, biochemical phenotype…) and taking into account known
genotype-phenotype associations. For some specific syndromes (e.g. Leigh
syndrome, Alpers syndrome, Sengers syndrome, MNGIE, LBSL, LTBL, etc…) it is
possible to perform genetic analysis without preliminary biochemical studies.
New technologies for sequencing (NGS) and bioinformatics tools allow to analyze
rapidly and at low cost the coding regions of human genome, making the
sequencing of a selected group of genes (gene panel) or all of them (whole
exome) possible. To better manage molecular exams, given the high genetic
heterogeneity of mitochondrial diseases, in our Unit we have set up different
gene-panels for the analysis of tens of genes in a single run, using an NGS
platform.
The available gene-panels include: Complex I deficiency genes; Complex II deficiency genes; Complex III deficiency genes; Complex IV deficiency genes; Complex V deficiency genes; Coenzyme Q10 deficiency genes; FeS-dependent complexes genes; PDH deficiency genes; mtDNA deletions/depletion genes; Multiple deficiency genes; Optic atrophy genes.
In addition to the sequencing of nuclear genes, recently we have extended the use of NGS technics to the analysis of mtDNA (ordinarily performed by direct sequencing and Southern blot). The advantages of this approach are a higher sensibility and accuracy together with a reduction in cost and time for analysis.
Ragged red fibers are a typical hallmark of mitochondrial myopathies
Double histochemical staining for succinate dehydrogenase and cytochrome c oxidase: the “ragged blue fiber” is equivalent to a ragged-red fiber: increased staining indicates abnormal proliferation of mitochondria
Numerous muscle fibers stain negative (white) to the histochemical staining specific to cytochrome c oxidase
Ultrastructural abnormalities: electron microscopy examination shows mitochondria which are altered in shape, size and internal structure
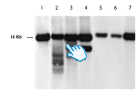
Southern blot mtDNA analysis: 1 and 7 wt; 2 and 3 multiple deletions; 4 macrodeletion; 5 and 6 depletion